Color is so much more than meets the eye. It has emotional elements that impact product positioning and consumer preferences. It is also a chemical variable that affects not only appearance but also the functionality, processing, and cost of plastic products. Processors need a solid understanding of how color is created in plastic and an appreciation for how valuable the assistance of a knowledgeable color supplier can be when color and plastics collide.
Basic color chemistry is well established. Colorists have a choice of using dyes, which are soluble and disperse in the polymer on a molecular level (like sugar in hot water), or pigments, which are insoluble solids that must be dispersed in the polymer matrix. Dyes make it easier to achieve bright, clear, transparent colors, and are the best solution for coloring transparent resins. Pigments, being solids, are better for deep, saturated, opaque or transparent colors. In recent years, there has been a dramatic move toward use of organic dyes or pigments and away from inorganic colorants that rely on regulated metals like cadmium or lead chromates.
Designing a color formulation is about more than what looks good. Colorant selection requires careful consideration of the resin type and source, application requirements—such as weatherability, heat stability, and mechanical properties—and effects on processing.
Inorganic pigments are finely ground complexes of metals. They have outstanding lightfastness, but have poor color strength, high density, and lower intensity and chromaticity than organics. Common inorganic pigments include oxides of metals like iron, titanium, or cobalt; inorganic salt complexes, such as ultramarine blue; and sulfides or sulfates of zinc, barium, and cerium.
Organic pigments, on the other hand, are finely ground particles of reactor-synthesized organic molecules. Depending on their base chemistry and functional groups, they can be widely variable in their light and temperature stability. The different classes of organic pigments are too numerous to mention here, but they are available in a wide variety of colors. Many offer high levels of brightness and tint strength. Particle sizes vary, with smaller particles being more transparent and having higher tint strength, and larger ones being more opaque with lower tint strength.
RESIN TYPE MATTERS
What kind of color you use in a given application will be determined by the basic structure of the polymer. Even similar polymers, produced by different companies or in different regions of the world, can vary in base color so that they respond to colorants differently. A particular color that works well in a polycarbonate produced in North America, for instance, may yield a totally different result if it is used in material sourced in Europe or Asia.
The molecular structure of the polymer determines its processing characteristics, which in turn will influence color choices. A crystalline resin like PP has a white semi-opaque appearance due to the mixture of ordered and disordered domains, which bend light differently. Amorphous (non-crystalline) resins are almost all disordered so that light passes straight through. This is why they are transparent. Naturally, extremely transparent colors are only possible in amorphous resins.
Crystalline resins have a very sharp melting point and require significant energy (heat) input before they undergo a phase change and melt to a low viscosity. For this reason, colors used in crystalline polymers may need to be more heat stable than those used in other materials. This usually suggests the use of inorganic pigments that are more stable at high-temperatures. It also explains why colors may change when regrind is blended with virgin resin. The color in the regrind, which has a longer heat history, degrades.
The earlier that color is considered in the product development cycle, the greater the opportunity to avoid needless waste of time and expense. A knowledgeable vendor can provide laboratory analysis, flow simulation, and performance testing to identify and avoid failure risks.
Amorphous resins, on the other hand, have a glass-transition temperature above which they start to soften until their melt viscosity is low enough to flow. Amorphous resins have significantly more free volume than crystalline resins, so they are more receptive to dyes, which are able to stay in solution without surface blooming or tool plate-out.
The selection and ultimate performance of colorants also depends on whether a plastic resin is a homopolymer (involving a single polymeric chemistry) or a copolymer, which involve two types of chemistry that respond differently. A homopolymer may be either crystalline or amorphous, but appropriate colorants will still be homogeneously distributed though out the resin.
Copolymers, on the other hand can be either random (having uniform phases) or block copolymers, which segregate into aggregates of the two chemistries. Impact-modified materials like ABS, HIPS and acrylic, for instance, contain cross-linked rubber particles. Colorants cannot get into the rubber phase and the rubber changes how light moves through the toughened resin, making it appear very white. Rubber alloys like super-tough nylon involve very different chemistries between the nylon and rubber, so they can be very difficult to color, with limited options.
The solubility of a resin (the way in which hydrogen atoms are bonded and the polarity of the material) will affect how well dyes will stay in the polymer. Dyes that are soluble in PS or ABS will slowly migrate to the surface of nylon. There are also pigments that will become soluble in the wrong resin. Consequently, if a dye is to be used to color plastic, it is very important that it be compatible with the resin.
Still another factor to consider is the refractive index of the resin, which defines the angle that light is bent as it passes through a polymer. Aliphatic resins (PE, PP, and EVA) have low refractive indices, while aromatic resins (PS, PC, PSU, PET) have high refractive indices. When resins of different refractive indices are mixed together, to improve impact strength or chemical resistance, for instance, light does not have a single path to follow, so it appears to scatter, making the material look opaque white. Again it is important to understand factors like these that limit the colorability of a resin.
The reactivity of a pigment can dramatically degrade the stability of the polymer. TiO2 will reduce the thermal stability of polyesters and polycarbonates, while iron salts will do the same for PVC. Improper TiO2 selection reduces the UV stability of polyethylene. Likewise, the reactivity of a polymer end group can alter the chemistry of certain colorants and lead to a color change. Amides, for instance, can affect chlorine- or bromine-containing pigments, while anhydrides can adversely affect metal salts of vat dyes.
In all cases, the functionality of the resin should be considered first and then color can be worked around the application.
IMPACT ON PROPERTIES, PROCESSING
Colorants can affect physical properties of plastic components. Color particles can function as a void in the polymer matrix, creating a tear point or causing poor interfacial adhesion. In fact, inorganic colorants can actually notch fiberglass reinforcements. All of these conditions can limit tensile, elongation, and impact properties in many applications. The best pigments for outdoor weatherability, for instance, actually are likely to reduce these properties the most. Proper color choice and formulation, however, can limit this reduction to no more than a 10%.
Living-hinge properties, which require a combination of toughness and orientation, are seriously affected by colorants. Hard voids can reduce the flex life of a hinge. If the masterbatch supplier knows upfront that toughness is going to be an issue, carrier resins can be formulated with additional tougheners to compensate for the loss. In many cases, it may even be possible to improve impact resistance.
Certain combinations of pigments, dyes, and resins can result in a phenomenon called phototendering, in which products lose strength and flexibility from exposure to sunlight. Some of the more challenging combinations included uncoated TiO2 or iron salts in polyolefins and standard coated grades of TiO2 in polycarbonate.
Dyes and pigments must be selected with awareness of their effects on flow, physical properties, shrinkage, warpage, and heat or light stability.
The thermal stability of “touchy” resins can also be compromised by trace metals often found in metallized dyes, lake pigments, and non-synthetic inorganic pigments. Of particular concern are ultramarine blue in acetal, manganese violet in ABS, and iron oxide in PVC. In order to achieve the most robust formulations, the best approach may be to formulate for light and thermal stability first and then, if necessary, compromise on color.
Rheological effects of colorants are often investigated only after there is a problem, but at that point formulation and tooling changes can be very expensive. Therefore it is important to consider the effect certain color-related materials can have on the melt viscosity of a resin. For instance, the high structure of carbon black and calcium carbonate will cause them to act like fillers and increase melt viscosity. Solvent dyes can reduce viscosity, as can some carriers used in liquid colors. And any colors or additives that cause degradation reactions in the polymer, as discussed above in the context of reactivity, can also reduce viscosity.
Processing temperature is another consideration. When running a material that requires high temperatures or high shear, it is best to avoid colorants that lack stability at elevated temperatures—inexpensive salt-based pigments, for instance. The color may degrade, sometimes causing light or dark streaks in the part, or an adverse reaction with the polymer may reduce important mechanical properties in the end product.
A related issue is dwell time. Even if the processing temperatures do not seem too high for the colorant, extended exposure—due to oversized barrels with too much capacity, for instance—may have the same degrading effect. If scrap regrind is returned to the process, the additional heat history may cause the color to degrade. As a general rule, the less expensive organic pigments tend to be the least stable, so a lowest cost formulation may not be the most robust, and any raw-material savings will be wasted in lower yields.
Many types of pigments can affect rates of shrinkage and warpage. General-purpose phthalocyanine green or blue, for instance, has a crystalline structure that can increase nucleation in semi-crystalline resins like PP and cause unexpected shrinkage. Computerized flow-simulation programs can predict the effect so that formulations can be adjusted before you notice that the green parts and the white parts are not the same size.
Pigments can affect the way that a resin will reheat, hold heat, or transfer heat. For instance, carbon black absorbs heat quickly and transfers it well, while ceramic (mixed-metal oxide) pigments tend to hold heat for a long time and special grades reflect heat. Aluminum pigments transfer heat extremely well. All of these effects can impact cycle time, part dimensions, and secondary processes like welding. If a job is trialed and quoted based on cycle times achieved with natural (uncolored) resin, it may become unprofitable due to changes in thermal conductivity caused by colorants.
THINK ABOUT COLOR EARLY
Costs balloon when color decisions are made late in the product development process. Maximum value for plastics products cannot be realized unless color and additive decisions are integrated into the design process early, before tooling is cut and certainly before a molding job is quoted. Attempting to cut costs by simply ordering a less expensive colorant is asking for trouble. On the other hand, a brief conversation with a knowledgeable colorant supplier can go a long way toward avoiding problems. The supplier will be able lead you to formulations that are both robust and cost-effective.
To help bring color into the product development process as early as possible, processors and their customers would be well advised to take advantage of color consultation services offered by larger color suppliers. Laboratory analysis, flow simulation, and performance testing can identify failure risks so they can be avoided before costly commitments of resources are made.
by: By Justin Christie, Clariant Masterbatches
Saturday, October 30, 2010
Thursday, October 28, 2010
How to work effortlessly
Sometimes work can be a drag. You get caught up in trying to be more productive and suddenly your life turns into a series of to-do lists. You gauge your measure of success by how much you accomplish. You even determine how happy you allow yourself to be by how much you’ve gotten done in the day.
We spend a lot of time trying to find ways to be more productive. To do things faster, better, cheaper and spend our time more effectively. But instead of just doing more in less time, maybe we should focus on actually enjoying the work we’re doing instead.
Here are 8 ways to make work seem like less of a chore and more like a gift:
1. Follow your natural rhythms. A lot of the time I resent working is because I’m trying to force myself to do something I don’t feel like doing. Naturally there will always be some things you’re not crazy about doing (like cleaning the toilet). But how often do you force yourself to work more, when you really want to relax? When you force yourself to work when you’ve promised yourself a break, you’ll likely just end up distracting yourself with other things and put off working. Then you get stressed and end up resenting work. Instead, follow your natural rhythms. When you feel like working, work. When you don’t, don’t. Don’t over complicate things.
2. Do, don’t think. I’m going to stay true to this point and not think about writing something elaborate. Just do, stop thinking about it. Fail, make corrections later.
3. Don’t put sugar in your tank. You wouldn’t put sugar in your gas tank right? It doesn’t make much sense to fill your body up with unhealthy fuel either. If you don’t have the energy to get the work you need to done, work will feel forced.
4. Remove hidden roadblocks. What’s making you avoid working? What’s making your work seem like drudgery rather than joyful? It might have something to do with your beliefs about yourself. Maybe you believe you’re not good enough, smart enough or don’t have enough experience. Question your beliefs about what you can and can’t do.
5. Only do your best. Work can easily become a chore when you’re trying to constantly be perfect. The truth is, some of your ideas might not be so great. Others will be mind blowing. If you can accept that and just do your best, you stop judging yourself. Guess what it feels like when you’re no longer picking over everything you do with a fine toothed comb? It feels extremely liberating. It feels like you can actually enjoy your experience, rather than worrying about how everything is going to turn out. That is working effortlessly.
6. Act from your gut. When you think you have a great idea, believe it. Follow it. Chase it until you’re out of breath and can barely hold yourself up. Because if you don’t trust yourself, you’ll regret it later. The best way to live is to follow your intuition and trust life. If nothing else, trust yourself. Because if you can’t trust yourself, how can you trust your mistrust? That’s not very smart is it?
7. Focus on what matters. Our minds are constantly pulling us in different directions. We have to wash the cat, buy more apple cinnamon oatmeal, finish writing that resignation letter to your no-longer-boss at your dead-end job, and all sorts of other things. We have a tendency to follow what’s urgent instead of what’s important. In order to get the important things done, we have to be ruthless at removing distractions. If it takes bringing a laptop (or notepad) to a cafe to write your grandiose novel, then do that. Avoid the vacuum of minutiae urgency. Remove all distractions so you can focus on the important things. I would much rather spend 4 hours working on an important project, then 4 hours spinning my wheels and scratching my head trying to figure out what I did today.
8. Refuse to do what you don’t want to do. I often avoid working because I’m trying to do things I think I “should do.” I think I should read more because it will make me smarter. I think I should buy new clothes because it will make me cooler. I think I should work on this project because it will be good for my resume. Forget what you think you should do (except maybe… paying your rent). Do what you want to do. Other people will understand. In fact, they’ll probably envy you.
We spend a lot of time trying to find ways to be more productive. To do things faster, better, cheaper and spend our time more effectively. But instead of just doing more in less time, maybe we should focus on actually enjoying the work we’re doing instead.
Here are 8 ways to make work seem like less of a chore and more like a gift:
1. Follow your natural rhythms. A lot of the time I resent working is because I’m trying to force myself to do something I don’t feel like doing. Naturally there will always be some things you’re not crazy about doing (like cleaning the toilet). But how often do you force yourself to work more, when you really want to relax? When you force yourself to work when you’ve promised yourself a break, you’ll likely just end up distracting yourself with other things and put off working. Then you get stressed and end up resenting work. Instead, follow your natural rhythms. When you feel like working, work. When you don’t, don’t. Don’t over complicate things.
2. Do, don’t think. I’m going to stay true to this point and not think about writing something elaborate. Just do, stop thinking about it. Fail, make corrections later.
3. Don’t put sugar in your tank. You wouldn’t put sugar in your gas tank right? It doesn’t make much sense to fill your body up with unhealthy fuel either. If you don’t have the energy to get the work you need to done, work will feel forced.
4. Remove hidden roadblocks. What’s making you avoid working? What’s making your work seem like drudgery rather than joyful? It might have something to do with your beliefs about yourself. Maybe you believe you’re not good enough, smart enough or don’t have enough experience. Question your beliefs about what you can and can’t do.
5. Only do your best. Work can easily become a chore when you’re trying to constantly be perfect. The truth is, some of your ideas might not be so great. Others will be mind blowing. If you can accept that and just do your best, you stop judging yourself. Guess what it feels like when you’re no longer picking over everything you do with a fine toothed comb? It feels extremely liberating. It feels like you can actually enjoy your experience, rather than worrying about how everything is going to turn out. That is working effortlessly.
6. Act from your gut. When you think you have a great idea, believe it. Follow it. Chase it until you’re out of breath and can barely hold yourself up. Because if you don’t trust yourself, you’ll regret it later. The best way to live is to follow your intuition and trust life. If nothing else, trust yourself. Because if you can’t trust yourself, how can you trust your mistrust? That’s not very smart is it?
7. Focus on what matters. Our minds are constantly pulling us in different directions. We have to wash the cat, buy more apple cinnamon oatmeal, finish writing that resignation letter to your no-longer-boss at your dead-end job, and all sorts of other things. We have a tendency to follow what’s urgent instead of what’s important. In order to get the important things done, we have to be ruthless at removing distractions. If it takes bringing a laptop (or notepad) to a cafe to write your grandiose novel, then do that. Avoid the vacuum of minutiae urgency. Remove all distractions so you can focus on the important things. I would much rather spend 4 hours working on an important project, then 4 hours spinning my wheels and scratching my head trying to figure out what I did today.
8. Refuse to do what you don’t want to do. I often avoid working because I’m trying to do things I think I “should do.” I think I should read more because it will make me smarter. I think I should buy new clothes because it will make me cooler. I think I should work on this project because it will be good for my resume. Forget what you think you should do (except maybe… paying your rent). Do what you want to do. Other people will understand. In fact, they’ll probably envy you.
Saturday, October 23, 2010
How to deal with work stress ??
Work stress bringing you down? In today’s highly competitive environment, it’s tough to avoid work-related stress. In a worst-case scenario, it can cause health problems or tension at home and performance issues at work. Psychologists and counselors advise that employees should identify the cause of their stress and tackle it head on. But often, there’s no single cause and it’s a combination of factors. In that case, you need to improve your ability to cope with stress.
Here are some tips to follow in your daily routine, which can help you do that.
It’s common for many of us to stay stuck to our desks for hours on end because we feel we can’t afford to take a break. But counselors say this hurts us in the long-run, because our minds can’t function efficiently non-stop.
They advise taking a three- to five-minute break every one or two hours. It could be as simple as walking to the office water cooler, or getting up from your desk to stretch your muscles. “Just taking a break and walking around refreshes you,” says T.V. Mohandas Pai, director of human resources, education, research and administration at Infosys Technologies Ltd.
When you return from your walk, you’ll find yourself being more productive.
2. The lunch break
Lunch time is likely your longest break during the work day, so don’t waste it.
If you are not in the habit of taking regular lunch, set a reminder in your Outlook or mobile phone to do so. Even on hectic days when you might be tempted to skip lunch, don’t do it.
“If possible, get up and get away from your desk when you’re eating, because otherwise you’re staying in that high-stress area for a long time,” says Karuna Bhaskar, a counseling psychologist at 1to1help.net Pvt. Ltd., a counseling firm in Bangalore.
3. A hobby and a friend help
Try and squeeze in a 10- to 15-minute break once every three hours and use that time to pursue activities that give you pleasure. “Have a cup of coffee with somebody else. Crack some jokes,” says Ms. Bhaskar. If you are an artist, you could try sketching, or go play table tennis if your office has that facility. Doing something different from what you do for the rest of the day can make your break more meaningful, says Ms. Bhaskar. If you can’t afford to get away from your desk, try listening to soothing music.
4. The right foods
When we are stressed out, some of us tend to eat more. Often, we turn to junk food like chips or cookies or sweets. But while sugar can provide a short-term rush, it can leave you feeling deflated not to mention adding to your weight.
Do yourself a favor by keeping some healthy snacks around. These could include dried fruits, peanuts, cut-up carrots and cucumbers.
5. Nice surroundings help
If you have a desk or a cubicle, it’s almost like your home during the work day. Try and keep your work-space tidy – that will not only allow you to find things more easily but also lend a sense of calmness and of having things under control.
Try adding a personal touch to the workplace, like a family photo –something which reminds you what all the hard work is for.
6. Time management
This may sound clichéd but it works!
Often, we get stressed because we’re rushing to complete our tasks and meet our deadlines. Maybe you have too much on your plate but more likely, the lack of time is because of poor time-management. Planning in advance can help. “You need to prioritize your work. Make a list of the most important jobs you have to deal with. Learn to delegate,” says Monica Chib, senior consultant in psychiatry at Apollo Hospitals.
One idea could be to come to work 30 minutes before everyone else and use that quiet time for thinking and planning for the day. Stay late at work if you need to once in a while, but “don’t carry forward (work) to the last day,” says Mr. Pai.
7. Deep breathing
Sometimes when we are seriously stressed out, we are so focused on our work that our breathing becomes shallow. Take a minute to breathe deeply. Put your hand on your stomach and when you inhale deeply, your abdomen should come out. When you exhale, it should go in. Do this a few times and you will feel relaxed almost immediately.
8. Get a life
Believe it or not, what you do outside work has a huge impact on how you feel at work.
If you simply come home, eat dinner and start working again, you will never completely relax. Instead, do what Mr. Pai does. He’ll put in 10- or 12-hour days if needed to finish his tasks but “when I go home, I forget about work,” he says.
If possible, make plans to do something once or twice a week, say dinner or a movie. It gives you something to look forward to during your work days. Make plans for the weekend. Mr. Pai recommends taking up a cause or an interest outside of work, such as volunteering or joining an ecology club. This helps provide “a sense of accomplishment outside of work,” says Mr. Pai, and “a reason for existence.”
9. Exercise
When you are stressed, your body tenses up as if it’s about to get into a fight. The body can’t distinguish between mental and physical stress, says Ms. Bhaskar.
Exercise can help burn off that energy, thus easing your tension.
So make it a point to exercise often, even if it’s for a short period of time. It will also generally make you happier because exercise releases feel-good hormones. As an added bonus, regular exercise gives you a sense of achievement from having accomplished what you had planned to do.
10. Better lifestyle choices
Sometimes people turn to smoking or excessive drinking as stress-busters, but these cause much bigger problems in the long run. Instead, individuals should make lifestyle choices which make them physically fit, which in turn, helps them cope with stress better, says Ms. Chib of Apollo. “Get adequate hours of sleep which is something that people these days don’t really get,” she says.
Here are some tips to follow in your daily routine, which can help you do that.
It’s common for many of us to stay stuck to our desks for hours on end because we feel we can’t afford to take a break. But counselors say this hurts us in the long-run, because our minds can’t function efficiently non-stop.
They advise taking a three- to five-minute break every one or two hours. It could be as simple as walking to the office water cooler, or getting up from your desk to stretch your muscles. “Just taking a break and walking around refreshes you,” says T.V. Mohandas Pai, director of human resources, education, research and administration at Infosys Technologies Ltd.
When you return from your walk, you’ll find yourself being more productive.
2. The lunch break
Lunch time is likely your longest break during the work day, so don’t waste it.
If you are not in the habit of taking regular lunch, set a reminder in your Outlook or mobile phone to do so. Even on hectic days when you might be tempted to skip lunch, don’t do it.
“If possible, get up and get away from your desk when you’re eating, because otherwise you’re staying in that high-stress area for a long time,” says Karuna Bhaskar, a counseling psychologist at 1to1help.net Pvt. Ltd., a counseling firm in Bangalore.
3. A hobby and a friend help
Try and squeeze in a 10- to 15-minute break once every three hours and use that time to pursue activities that give you pleasure. “Have a cup of coffee with somebody else. Crack some jokes,” says Ms. Bhaskar. If you are an artist, you could try sketching, or go play table tennis if your office has that facility. Doing something different from what you do for the rest of the day can make your break more meaningful, says Ms. Bhaskar. If you can’t afford to get away from your desk, try listening to soothing music.
4. The right foods
When we are stressed out, some of us tend to eat more. Often, we turn to junk food like chips or cookies or sweets. But while sugar can provide a short-term rush, it can leave you feeling deflated not to mention adding to your weight.
Do yourself a favor by keeping some healthy snacks around. These could include dried fruits, peanuts, cut-up carrots and cucumbers.
5. Nice surroundings help
If you have a desk or a cubicle, it’s almost like your home during the work day. Try and keep your work-space tidy – that will not only allow you to find things more easily but also lend a sense of calmness and of having things under control.
Try adding a personal touch to the workplace, like a family photo –something which reminds you what all the hard work is for.
6. Time management
This may sound clichéd but it works!
Often, we get stressed because we’re rushing to complete our tasks and meet our deadlines. Maybe you have too much on your plate but more likely, the lack of time is because of poor time-management. Planning in advance can help. “You need to prioritize your work. Make a list of the most important jobs you have to deal with. Learn to delegate,” says Monica Chib, senior consultant in psychiatry at Apollo Hospitals.
One idea could be to come to work 30 minutes before everyone else and use that quiet time for thinking and planning for the day. Stay late at work if you need to once in a while, but “don’t carry forward (work) to the last day,” says Mr. Pai.
7. Deep breathing
Sometimes when we are seriously stressed out, we are so focused on our work that our breathing becomes shallow. Take a minute to breathe deeply. Put your hand on your stomach and when you inhale deeply, your abdomen should come out. When you exhale, it should go in. Do this a few times and you will feel relaxed almost immediately.
8. Get a life
Believe it or not, what you do outside work has a huge impact on how you feel at work.
If you simply come home, eat dinner and start working again, you will never completely relax. Instead, do what Mr. Pai does. He’ll put in 10- or 12-hour days if needed to finish his tasks but “when I go home, I forget about work,” he says.
If possible, make plans to do something once or twice a week, say dinner or a movie. It gives you something to look forward to during your work days. Make plans for the weekend. Mr. Pai recommends taking up a cause or an interest outside of work, such as volunteering or joining an ecology club. This helps provide “a sense of accomplishment outside of work,” says Mr. Pai, and “a reason for existence.”
9. Exercise
When you are stressed, your body tenses up as if it’s about to get into a fight. The body can’t distinguish between mental and physical stress, says Ms. Bhaskar.
Exercise can help burn off that energy, thus easing your tension.
So make it a point to exercise often, even if it’s for a short period of time. It will also generally make you happier because exercise releases feel-good hormones. As an added bonus, regular exercise gives you a sense of achievement from having accomplished what you had planned to do.
10. Better lifestyle choices
Sometimes people turn to smoking or excessive drinking as stress-busters, but these cause much bigger problems in the long run. Instead, individuals should make lifestyle choices which make them physically fit, which in turn, helps them cope with stress better, says Ms. Chib of Apollo. “Get adequate hours of sleep which is something that people these days don’t really get,” she says.
what to include in resignation letter !!
The more important question to be asking is what not to include, say the experts. "Less is always more," says Roy Cohen, a Manhattan-based career counselor and executive coach. "This is not the time to set the record straight. Know that it's a small world." By leaving on the best note possible, you also keep open the option for a return to the company should your circumstances change.
By leaving on the best note possible, you keep open the option for a return to the company should your circumstances change.
.Rather than airing your grievances with the company, you should set a positive tone from the start, says Marilyn Puder-York, a psychologist and executive coach in the New York metro area. One way to do this is to include a sentence or two at the top that shows your appreciation for the opportunity to work at the company and the experience it has given you.
Small courtesies are also important. This includes giving enough notice: a minimum of two weeks but preferably one month, says Ms. Puder-York, who has seen people give as much as six months, a move that she wouldn't recommend. "You lose a lot of power and credibility in six months," she says. Your preferred last day should also be included in the letter.
Both Mr. Cohen and Ms. Puder-York recommend that you don't list reasons for your resignation, no matter how tempting it might seem. "Once you've made the decision to leave, the reasons are superfluous," says Mr. Cohen. One option is to include the following sentence at the end of your letter: "I would be happy to discuss my reasons for resigning as well as any particular support I can give you during the transition," suggests Ms. Puder-York.
"Make the letter clear, direct and simple," she says. "You should always wait to give additional information in a verbal discussion. The letter ends up in your file. You don't know where it is going to go."
At some companies, a formal resignation letter may not even be necessary, says Ms. Puder-York. But she still recommends submitting one, equating it with the increasingly rare written thank-you note. "It is the smart, respectful thing to do, and it's a gracious thing to do if you do it well," she says.
By leaving on the best note possible, you keep open the option for a return to the company should your circumstances change.
.Rather than airing your grievances with the company, you should set a positive tone from the start, says Marilyn Puder-York, a psychologist and executive coach in the New York metro area. One way to do this is to include a sentence or two at the top that shows your appreciation for the opportunity to work at the company and the experience it has given you.
Small courtesies are also important. This includes giving enough notice: a minimum of two weeks but preferably one month, says Ms. Puder-York, who has seen people give as much as six months, a move that she wouldn't recommend. "You lose a lot of power and credibility in six months," she says. Your preferred last day should also be included in the letter.
Both Mr. Cohen and Ms. Puder-York recommend that you don't list reasons for your resignation, no matter how tempting it might seem. "Once you've made the decision to leave, the reasons are superfluous," says Mr. Cohen. One option is to include the following sentence at the end of your letter: "I would be happy to discuss my reasons for resigning as well as any particular support I can give you during the transition," suggests Ms. Puder-York.
"Make the letter clear, direct and simple," she says. "You should always wait to give additional information in a verbal discussion. The letter ends up in your file. You don't know where it is going to go."
At some companies, a formal resignation letter may not even be necessary, says Ms. Puder-York. But she still recommends submitting one, equating it with the increasingly rare written thank-you note. "It is the smart, respectful thing to do, and it's a gracious thing to do if you do it well," she says.
Saturday, October 16, 2010
Impact of miniral fillers on Extruded films
::Minerals Adding Value to Polyethylene Film Extrusion: Process Optimisation and Economics::
Minerals have, historically, been added as extenders and low-cost fillers for polyolefins, PVC and others polymers. Recent studies have shown that minerals, specifically ground calcium carbonates, alter processing cycle times and improve physical properties of polyolefins. To verify these findings we studied the thermal characteristics for blown LLDPE film with mineral additives. Masterbatches were prepared with various calcium carbonate grades, and surface-modified calcined clays. Laboratory-scale films were produced, and processing temperatures monitored. Improved thermal properties of the resultant films gave processing benefits, including output-rate and bubble stability. Laboratory findings were verified at a waste bag production facility. Using a full-scale line, film output speeds were increased by 20 % with the addition of 20 wt.% calcium carbonate. Bag conversion was also enhanced.
The physical properties of the laboratory films were measured. With the addition of surface-modified calcium carbonate improvements were seen in impact strength, tear strength and coefficient of friction. The use of water-based inks was assessed by water contact angle measurement. Addition of minerals increased contact angle (cosq) proportional to concentration.
additives were found to give a number of cost benefits: processing rates could be increased; films could be downgauged; expensive antiblocks could be excluded; and surface treatment time before printing could be reduced.
In any multiphase system, each component has an influence on the processing and performance of the product. Blown polyolefin films are affected by changes in processing equipment, polymer type and additives. By including functional mineral additives to the polymer melt, significant changes can be made to these systems. Heating and cooling of blown film are influenced by the fundamental properties of components within the melt. By studying the thermal processing of each component and equating these to processing changes, the effect on processing can be understood. Previous data have shown that minerals require specific particle size distributions, a maximum particle size, and surface-modification to compatibilize the mineral and polymer.
It has found that hexene, octene and butene LLDPE blown films had increased strength with the addition of up to 20 wt.% surface-modified gcc (ground calcium carbonate). Extrusion outputs increased by 22 % , 39 % and 47 % respectively with 20 wt.% gcc. Cooling rates were improved due to the greater thermal conductivity of the mineral compared with that of the bulk polymer. There may also be changes in extensional viscosity with certain minerals. The difficulties of processing linear resins compared with LDPE are well documented, and it is here that the benefits of mineral addition are most significant.
Organic coated calcium carbonates of fine particle size are already in use commercially to improve the processing of polyethylenes, and to aid bubble stability. The study described here encompasses particle size, loading and shape effects of minerals in LLDPE blown film. During processing, film temperatures were monitored and equated to the thermal properties of the matrices.
Physical properties of thin-gauge (40 mm) films were measured to assess end-use viability. Surface properties were characterised by dyne solutions. These properties were correlated with flexographic testing of print properties and surface tension measurements of corona-treated films. Surface roughness was also measured.
Materials and Preparation
Coated calcium carbonates are often favoured in polyethylene films because processing can be improved whilst maintaining a good balance of mechanical properties. Indeed some properties, such as film toughness, are significantly enhanced. For this study, other minerals are included to provide additional, comparative data. They are not, as far as we are aware, currently in commercial use at these concentrations.
All minerals were IMERYS materials, and were either commercial or experimental grades. Details are given in Table 1. The polymer was Exxon Escorene LL3001.32, a hexene copolymer LLDPE film grade, MFI 1.0 g 10 min-1.
Table 1: Legend and physical data for mineral fillers
Laboratory Trials
Masterbatches of coated calcium carbonate (60 wt.%) and other minerals (40 wt.%) in LLDPE were prepared on an APV Baker Perkins 2030 twin screw compounder. Die temperature was 200°C, and extrusions were run at a constant torque of 50%. Each mineral was let down in virgin resin on a Killion KN150 38 mm laboratory blown film line to produce 20 wt.% mineral-filled films. Film gauge was 0.025 mm and 250 mm layflat. Films K1 and K2 contained the unmodified and silane-treated calcined kaolins respectively and C1 to C4 contained surface-treated gccs. Additionally, films with 0 (U), 10 (C2/10), 30 (C2/30), 40 (C2/40) and 50 wt.% (C2/50) gcc were prepared.
Plant Trials
FilmLink 400 masterbatch (C2) was prepared at 60 wt.% on a Werner and Pfleiderer ZSK-40 twin screw compounder. Die temperature was 200°C, and extrusions were run at a constant torque of 85-90%, with an output of 100 kg/hr. The masterbatch was let down in virgin resin on a Battenfeld line (250 mm die), with a BUR of 2.4:1. The line had in-line conversion to produce dustbin liners (trash bag). Film gauge was 25 mm. Bags containing 0, 5, 10 and 20 wt.% FilmLink 400 were prepared.
Experimental
Film Processing 1, Laboratory Trials
Extrusion experiments were conducted on a Betol BK 32 laboratory-scale film line (80 mm diameter, 0.80 mm die gap, 450 mm max. layflat, 32 mm single screw extruder), to produce a 40 mm gauge film. The extruder and die temperatures were consistent throughout the experiment, as were the extrusion rate and line speed. Die temperature was set at 245 °C, extrusion rate was 5 kg hr-1 and line speed was 10 m min-1.
Film temperature was measured with a Minolta Land Cyclops 343 portable infrared (IR) thermometer. This measured the emitted IR radiation at 3.43 µm (3430 nm) wavelength, giving a true value for the surface temperature of the polymer. Emissivity differences were compensated for as necessary.
External air cooling was maintained by setting the air ring configuration and flow rate. The temperature of the film was recorded at various points along the bubble. As each filler was added, so the temperature changes at the points along the bubble were noted. A mean reading was displayed, collected over a ten second period. Three such readings were taken. The mean is quoted in the data.
Film Processing 2, Plant Trials
The integrated film/conversion line was optimised for maximum throughput under standard processing conditions, with the hexene LLDPE. Extrusion conditions were noted, and outputs from the film line, and bag converter measured. The FilmLink 400 masterbatch was then added, to give final concentrations of 5, 10 and 20 wt.%. Conditions and throughputs were again measured. Each bag was weighed and compensations made for this in the conversion data.
Physical Testing of Films
Drop dart impact strength for each film was measured with a Kayness D2085AB impact tester according to ASTM method D1709-916. Tear strength, by ASTM method D922-24a, was measured with a Thwing-Albert Elmendorf tear tester. Tensile strength was measured in the machine and transverse directions with a Flexsys T2000 tensometer using ASTM method D882. Blocking was assessed with a Kayness 9046 blocking force tester, according to ASTM D3354B. A Kayness sliding sled CoF tester was used to measure static and kinetic coefficients of friction, as specified in ASTM D1894-93.
Water wettability and surface tension measurements
Industrial dyne solutions were used to estimate the critical surface tension. The unfilled film and C2/10 to C2/50 were corona-treated. The treatment levels were adjusted to obtain surface tensions of 42 ± 2 dynes cm-1 for the unfilled film to conform with recognised levels for flexographic printing. Surface tensions of these were measured with an FTA200 dynamic instrument using the water contact angle method.
Flexographic printing
Print quality of each film was assessed by flexographic printing with water-based inks on an IGT F1 tester, with an antilox force of 50 N, printing force of 100 N and a print speed of 0.5 ms-1. Print density was measured with a Gretag D186 densitometer.
Surface Topographies
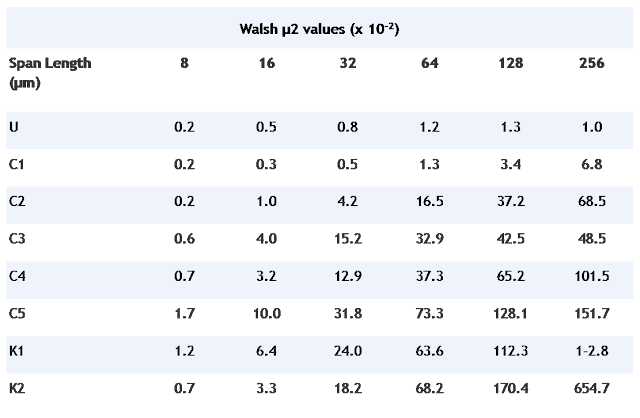
Surface profiles of an area (1 mm x 1 mm) of each film were mapped with a Talystep profilimeter. From these, surface roughness was calculated by Walsh analysis of the data.
Results and Discussion
Film Processing 1, Laboratory Trials
Figure 1 illustrates the effect of filler loading on the thermal profile of the bubble, with FilmLink 400 as the single mineral source. As addition levels increased, so the temperature at a specific point along the bubble decreased. This lead to a stepped lowering of the freeze-line. Changes in temperature profile with different particle size and mineralogy are shown in Figure 2.
Figure 1. The effect of mineral addition on the thermal profile of the bubble.
Figure 2. The effect of mineralogy on the thermal profile of the bubblePhysical Properties
Physical property data of the films (Table 3) show increases tear strength and drop dart impact in C2, compared with U. K1 and K2 both have higher tear strength, but a reduction in impact strength.
The impact strength of the films increased with the addition of C2, up to 30 wt.% (Figure 4), illustrating the extent of mineral reinforcement.
Table 2. Processing data from plant trials.
Figure 3. Concentration of Filler versus bag conversion rate
Figure 4. Impact strength of films containing C2 (FilmLink 400)
Film Processing 2, Plant Trials
The addition of high levels of C2 resulted in higher Elmendorf tear resistance. In the machine direction, additions of up to 40 wt.% increased tear strength. A maximum at 20 wt.% C2 was seen in the transverse direction.
Surface properties
C2/10 to C2/50 showed a trend of increasing surface tension with mineral concentration (Figure 7). These differences were also observed in print quality. Printing results for C2/10 to C2/50 revealed a trend of increased print density with mineral addition (Figure 8). K1 and K2 gave densities similar to those for C2/40 and C2/30 respectively.
The roughness of surface profiles was quantified by Walsh analysis, given in Table 4. The mineral-free film was very smooth with little surface variation at any span length. K1 and K2 both had highly irregular surfaces, indicated by the high µ2 values. C2/10 to C2/50 showed an increase in roughness with concentration.
Several factors are known to affect the thermal properties of blown films7. The most significant are the orientation and crystallinity of the polymer itself and the presence of additives. The former have not been considered in this paper, as the processing conditions of all the blown films were comparable. The latter was considered to be most influential in the current study. Due to the greater thermal conductivity of the added minerals, we can predict that the higher the loading of this phase, the greater will be the bulk heating or cooling effects. This was confirmed by the data in Figure 1, where the addition of increasing levels Filler served to successively lower the temperature of the melt at the die, and thus throughout the profile. The freeze-line dropped correspondingly.
The effect of particle size and shape on bubble cooling is shown in Figure 2. The addition of calcium carbonate led to lowering of the film temperature at any specific point. As the mean particle size increased, so the cooling effects became more apparent. The higher aspect ratio mineral, PoleStar 200R, behaved similarly to the mineral-free polymer. We concluded that particle shape influenced the rate of cooling.
A study of the electron micrographs of film cross-sections (C2 and K1) can be used to explain this effect. Orientation of PoleStar 200R was seen in the machine direction of polymer. Parallel alignment lowers particle-particle contact and increases the thermal conduction path through the polymer phase. As the polymer is known to be less thermally conductive than the mineral any such orientation will reduce its bulk cooling, when compared with an equivalent low aspect ratio mineral such as ground calcium carbonate. Voids or air pockets in the film can also have a marked influence on cooling. No voidage was seen in the C1 to C4 films. In K1, occasional debonding between polymer and mineral appeared as small voids. Entrained air leads to a lowering of the bulk conductivity, and hence reduces the rate of cooling. For 10 wt.% additions of minerals, the voidage was considered of secondary importance. If higher levels are included in the film, typically over 20 wt.%, they can become the dominant feature.
Laboratory observations were supported by plant trial data. Addition of FilmLink 400 masterbatch led to increased extruder throughput at equivalent screw speeds. Additionally the bubble cooled quicker, and the line could be run faster with a consequential rise in bag production from 100 to 116 bags per minute (with 20 wt.% addition).
Physical properties of the films (given in Table 3 and Figures 4 and 5) correlate with interfacial characteristics between the mineral and polymer. Surface-modified gcc, having a similar surface energy to polyethylene, will be compatible in the matrix. Impact resistance is therefore high8. Where the two systems are less compatible, as in K1 and K2, residual stresses may concentrate around the particles. Impact strength may thus be lower. Tear strength is believed to be dependent on reducing tear propagation. Mineral addition improved tear resistance in all systems. The fine particles prevented tear propagation by acting as a physical barrier to the rupture of the oriented polymer chains9. Marginally higher tear strength for K1 and K2 is explained in terms of interfacial debonding of mineral particles dissipating tear energy and physically preventing tear propagation.
Blocking force of the films decreased with increasing concentration from U to C2/50. Levels of 5 wt.% are sufficient to act as a suitable anti-blocking additive, although this could be considered as an additional benefit, rather than a significant functional change.
Print properties of the films are related to both surface energetics and surface roughness. As contact angle measurements will be influenced by surface topography, absolute conclusions cannot be drawn. Clearly, increased addition of a compatible, well-dispersed mineral, such as a surface-modified gcc (as in C2/10 to C2/50) improves water-based ink adhesion. This can be enhanced further by corona treatment. The disproportionately high surface topographies for K1 and K2 are considered to be due to poor interfacial adhesion of the mineral in the polymer matrix. Thus addition of less compatible minerals will also increase printablity, but may do so by increasing surface roughness.
The use of high loadings of calcium carbonate is beneficial both in terms of physical property improvements and print adhesion. Calcined kaolins are seldom used at concentrations of 20 wt.% in polyethylene film, and a comparison of physical properties with carbonate additives is inadvisable. Calcined kaolin is used widely in agricultural polyethylene films (at concentrations up to 10 wt.%) as an infra-red blocking agent, or at ppm levels as an antiblock10. By selection of mineral additives and concentrations, polyethylene films can be modified to the demands of the end user.
Summary
We have demonstrated that some physical and mechanical properties of linear low-density polyethylene (LLDPE) extruded and blown films are improved by the addition of surface-modified calcium carbonate. Thus by adding FilmLink 400 processing output may be increased by up to 50 %. Drop dart impact strength and tear resistance is increased significantly, giving the ability to down-gauge. Anti-blocking additives are not required, at recommended addition levels.
These findings were supported by plant trials using a standard film extruder with in-line bag conversion. Increased productivity and energy savings were observed.
Minerals have, historically, been added as extenders and low-cost fillers for polyolefins, PVC and others polymers. Recent studies have shown that minerals, specifically ground calcium carbonates, alter processing cycle times and improve physical properties of polyolefins. To verify these findings we studied the thermal characteristics for blown LLDPE film with mineral additives. Masterbatches were prepared with various calcium carbonate grades, and surface-modified calcined clays. Laboratory-scale films were produced, and processing temperatures monitored. Improved thermal properties of the resultant films gave processing benefits, including output-rate and bubble stability. Laboratory findings were verified at a waste bag production facility. Using a full-scale line, film output speeds were increased by 20 % with the addition of 20 wt.% calcium carbonate. Bag conversion was also enhanced.
The physical properties of the laboratory films were measured. With the addition of surface-modified calcium carbonate improvements were seen in impact strength, tear strength and coefficient of friction. The use of water-based inks was assessed by water contact angle measurement. Addition of minerals increased contact angle (cosq) proportional to concentration.
additives were found to give a number of cost benefits: processing rates could be increased; films could be downgauged; expensive antiblocks could be excluded; and surface treatment time before printing could be reduced.
In any multiphase system, each component has an influence on the processing and performance of the product. Blown polyolefin films are affected by changes in processing equipment, polymer type and additives. By including functional mineral additives to the polymer melt, significant changes can be made to these systems. Heating and cooling of blown film are influenced by the fundamental properties of components within the melt. By studying the thermal processing of each component and equating these to processing changes, the effect on processing can be understood. Previous data have shown that minerals require specific particle size distributions, a maximum particle size, and surface-modification to compatibilize the mineral and polymer.
It has found that hexene, octene and butene LLDPE blown films had increased strength with the addition of up to 20 wt.% surface-modified gcc (ground calcium carbonate). Extrusion outputs increased by 22 % , 39 % and 47 % respectively with 20 wt.% gcc. Cooling rates were improved due to the greater thermal conductivity of the mineral compared with that of the bulk polymer. There may also be changes in extensional viscosity with certain minerals. The difficulties of processing linear resins compared with LDPE are well documented, and it is here that the benefits of mineral addition are most significant.
Organic coated calcium carbonates of fine particle size are already in use commercially to improve the processing of polyethylenes, and to aid bubble stability. The study described here encompasses particle size, loading and shape effects of minerals in LLDPE blown film. During processing, film temperatures were monitored and equated to the thermal properties of the matrices.
Physical properties of thin-gauge (40 mm) films were measured to assess end-use viability. Surface properties were characterised by dyne solutions. These properties were correlated with flexographic testing of print properties and surface tension measurements of corona-treated films. Surface roughness was also measured.
Materials and Preparation
Coated calcium carbonates are often favoured in polyethylene films because processing can be improved whilst maintaining a good balance of mechanical properties. Indeed some properties, such as film toughness, are significantly enhanced. For this study, other minerals are included to provide additional, comparative data. They are not, as far as we are aware, currently in commercial use at these concentrations.
All minerals were IMERYS materials, and were either commercial or experimental grades. Details are given in Table 1. The polymer was Exxon Escorene LL3001.32, a hexene copolymer LLDPE film grade, MFI 1.0 g 10 min-1.
Table 1: Legend and physical data for mineral fillers
Laboratory Trials
Masterbatches of coated calcium carbonate (60 wt.%) and other minerals (40 wt.%) in LLDPE were prepared on an APV Baker Perkins 2030 twin screw compounder. Die temperature was 200°C, and extrusions were run at a constant torque of 50%. Each mineral was let down in virgin resin on a Killion KN150 38 mm laboratory blown film line to produce 20 wt.% mineral-filled films. Film gauge was 0.025 mm and 250 mm layflat. Films K1 and K2 contained the unmodified and silane-treated calcined kaolins respectively and C1 to C4 contained surface-treated gccs. Additionally, films with 0 (U), 10 (C2/10), 30 (C2/30), 40 (C2/40) and 50 wt.% (C2/50) gcc were prepared.
Plant Trials
FilmLink 400 masterbatch (C2) was prepared at 60 wt.% on a Werner and Pfleiderer ZSK-40 twin screw compounder. Die temperature was 200°C, and extrusions were run at a constant torque of 85-90%, with an output of 100 kg/hr. The masterbatch was let down in virgin resin on a Battenfeld line (250 mm die), with a BUR of 2.4:1. The line had in-line conversion to produce dustbin liners (trash bag). Film gauge was 25 mm. Bags containing 0, 5, 10 and 20 wt.% FilmLink 400 were prepared.
Experimental
Film Processing 1, Laboratory Trials
Extrusion experiments were conducted on a Betol BK 32 laboratory-scale film line (80 mm diameter, 0.80 mm die gap, 450 mm max. layflat, 32 mm single screw extruder), to produce a 40 mm gauge film. The extruder and die temperatures were consistent throughout the experiment, as were the extrusion rate and line speed. Die temperature was set at 245 °C, extrusion rate was 5 kg hr-1 and line speed was 10 m min-1.
Film temperature was measured with a Minolta Land Cyclops 343 portable infrared (IR) thermometer. This measured the emitted IR radiation at 3.43 µm (3430 nm) wavelength, giving a true value for the surface temperature of the polymer. Emissivity differences were compensated for as necessary.
External air cooling was maintained by setting the air ring configuration and flow rate. The temperature of the film was recorded at various points along the bubble. As each filler was added, so the temperature changes at the points along the bubble were noted. A mean reading was displayed, collected over a ten second period. Three such readings were taken. The mean is quoted in the data.
Film Processing 2, Plant Trials
The integrated film/conversion line was optimised for maximum throughput under standard processing conditions, with the hexene LLDPE. Extrusion conditions were noted, and outputs from the film line, and bag converter measured. The FilmLink 400 masterbatch was then added, to give final concentrations of 5, 10 and 20 wt.%. Conditions and throughputs were again measured. Each bag was weighed and compensations made for this in the conversion data.
Physical Testing of Films
Drop dart impact strength for each film was measured with a Kayness D2085AB impact tester according to ASTM method D1709-916. Tear strength, by ASTM method D922-24a, was measured with a Thwing-Albert Elmendorf tear tester. Tensile strength was measured in the machine and transverse directions with a Flexsys T2000 tensometer using ASTM method D882. Blocking was assessed with a Kayness 9046 blocking force tester, according to ASTM D3354B. A Kayness sliding sled CoF tester was used to measure static and kinetic coefficients of friction, as specified in ASTM D1894-93.
Water wettability and surface tension measurements
Industrial dyne solutions were used to estimate the critical surface tension. The unfilled film and C2/10 to C2/50 were corona-treated. The treatment levels were adjusted to obtain surface tensions of 42 ± 2 dynes cm-1 for the unfilled film to conform with recognised levels for flexographic printing. Surface tensions of these were measured with an FTA200 dynamic instrument using the water contact angle method.
Flexographic printing
Print quality of each film was assessed by flexographic printing with water-based inks on an IGT F1 tester, with an antilox force of 50 N, printing force of 100 N and a print speed of 0.5 ms-1. Print density was measured with a Gretag D186 densitometer.
Surface Topographies
Surface profiles of an area (1 mm x 1 mm) of each film were mapped with a Talystep profilimeter. From these, surface roughness was calculated by Walsh analysis of the data.
Results and Discussion
Film Processing 1, Laboratory Trials
Figure 1 illustrates the effect of filler loading on the thermal profile of the bubble, with FilmLink 400 as the single mineral source. As addition levels increased, so the temperature at a specific point along the bubble decreased. This lead to a stepped lowering of the freeze-line. Changes in temperature profile with different particle size and mineralogy are shown in Figure 2.
Figure 1. The effect of mineral addition on the thermal profile of the bubble.
Figure 2. The effect of mineralogy on the thermal profile of the bubblePhysical Properties
Physical property data of the films (Table 3) show increases tear strength and drop dart impact in C2, compared with U. K1 and K2 both have higher tear strength, but a reduction in impact strength.
The impact strength of the films increased with the addition of C2, up to 30 wt.% (Figure 4), illustrating the extent of mineral reinforcement.
Table 2. Processing data from plant trials.
Table 3. Physical properties of blown films.
Figure 3. Concentration of Filler versus bag conversion rate
Figure 4. Impact strength of films containing C2 (FilmLink 400)
Film Processing 2, Plant Trials
The addition of high levels of C2 resulted in higher Elmendorf tear resistance. In the machine direction, additions of up to 40 wt.% increased tear strength. A maximum at 20 wt.% C2 was seen in the transverse direction.
As the mineral content of the film increased, so blocking decreased (Figure 6).
Figure 5. Tear strength of films containing C2 (FilmLink 400)
Figure 6. Blocking properties of films containing C2 (FilmLink 400)
Surface properties
C2/10 to C2/50 showed a trend of increasing surface tension with mineral concentration (Figure 7). These differences were also observed in print quality. Printing results for C2/10 to C2/50 revealed a trend of increased print density with mineral addition (Figure 8). K1 and K2 gave densities similar to those for C2/40 and C2/30 respectively.
Figure 7. Surface tension of films containing FilmLink 400, measured by water contact angle (after corona treatment)
Figure 8. Water-based ink printing results for all films
The roughness of surface profiles was quantified by Walsh analysis, given in Table 4. The mineral-free film was very smooth with little surface variation at any span length. K1 and K2 both had highly irregular surfaces, indicated by the high µ2 values. C2/10 to C2/50 showed an increase in roughness with concentration.
Table 4. Walsh analyses of surface roughness.
Discussion
Several factors are known to affect the thermal properties of blown films7. The most significant are the orientation and crystallinity of the polymer itself and the presence of additives. The former have not been considered in this paper, as the processing conditions of all the blown films were comparable. The latter was considered to be most influential in the current study. Due to the greater thermal conductivity of the added minerals, we can predict that the higher the loading of this phase, the greater will be the bulk heating or cooling effects. This was confirmed by the data in Figure 1, where the addition of increasing levels Filler served to successively lower the temperature of the melt at the die, and thus throughout the profile. The freeze-line dropped correspondingly.
The effect of particle size and shape on bubble cooling is shown in Figure 2. The addition of calcium carbonate led to lowering of the film temperature at any specific point. As the mean particle size increased, so the cooling effects became more apparent. The higher aspect ratio mineral, PoleStar 200R, behaved similarly to the mineral-free polymer. We concluded that particle shape influenced the rate of cooling.
A study of the electron micrographs of film cross-sections (C2 and K1) can be used to explain this effect. Orientation of PoleStar 200R was seen in the machine direction of polymer. Parallel alignment lowers particle-particle contact and increases the thermal conduction path through the polymer phase. As the polymer is known to be less thermally conductive than the mineral any such orientation will reduce its bulk cooling, when compared with an equivalent low aspect ratio mineral such as ground calcium carbonate. Voids or air pockets in the film can also have a marked influence on cooling. No voidage was seen in the C1 to C4 films. In K1, occasional debonding between polymer and mineral appeared as small voids. Entrained air leads to a lowering of the bulk conductivity, and hence reduces the rate of cooling. For 10 wt.% additions of minerals, the voidage was considered of secondary importance. If higher levels are included in the film, typically over 20 wt.%, they can become the dominant feature.
Laboratory observations were supported by plant trial data. Addition of FilmLink 400 masterbatch led to increased extruder throughput at equivalent screw speeds. Additionally the bubble cooled quicker, and the line could be run faster with a consequential rise in bag production from 100 to 116 bags per minute (with 20 wt.% addition).
Physical properties of the films (given in Table 3 and Figures 4 and 5) correlate with interfacial characteristics between the mineral and polymer. Surface-modified gcc, having a similar surface energy to polyethylene, will be compatible in the matrix. Impact resistance is therefore high8. Where the two systems are less compatible, as in K1 and K2, residual stresses may concentrate around the particles. Impact strength may thus be lower. Tear strength is believed to be dependent on reducing tear propagation. Mineral addition improved tear resistance in all systems. The fine particles prevented tear propagation by acting as a physical barrier to the rupture of the oriented polymer chains9. Marginally higher tear strength for K1 and K2 is explained in terms of interfacial debonding of mineral particles dissipating tear energy and physically preventing tear propagation.
Blocking force of the films decreased with increasing concentration from U to C2/50. Levels of 5 wt.% are sufficient to act as a suitable anti-blocking additive, although this could be considered as an additional benefit, rather than a significant functional change.
Print properties of the films are related to both surface energetics and surface roughness. As contact angle measurements will be influenced by surface topography, absolute conclusions cannot be drawn. Clearly, increased addition of a compatible, well-dispersed mineral, such as a surface-modified gcc (as in C2/10 to C2/50) improves water-based ink adhesion. This can be enhanced further by corona treatment. The disproportionately high surface topographies for K1 and K2 are considered to be due to poor interfacial adhesion of the mineral in the polymer matrix. Thus addition of less compatible minerals will also increase printablity, but may do so by increasing surface roughness.
The use of high loadings of calcium carbonate is beneficial both in terms of physical property improvements and print adhesion. Calcined kaolins are seldom used at concentrations of 20 wt.% in polyethylene film, and a comparison of physical properties with carbonate additives is inadvisable. Calcined kaolin is used widely in agricultural polyethylene films (at concentrations up to 10 wt.%) as an infra-red blocking agent, or at ppm levels as an antiblock10. By selection of mineral additives and concentrations, polyethylene films can be modified to the demands of the end user.
Summary
We have demonstrated that some physical and mechanical properties of linear low-density polyethylene (LLDPE) extruded and blown films are improved by the addition of surface-modified calcium carbonate. Thus by adding FilmLink 400 processing output may be increased by up to 50 %. Drop dart impact strength and tear resistance is increased significantly, giving the ability to down-gauge. Anti-blocking additives are not required, at recommended addition levels.
These findings were supported by plant trials using a standard film extruder with in-line bag conversion. Increased productivity and energy savings were observed.
Subscribe to:
Posts (Atom)